Planarian Aging and Rejuvenation (2025)
Genetic and Genomic insights into planarian biology
Guo L
Annu Rev Genet. 2025 Aug 19
Journal Link pdf
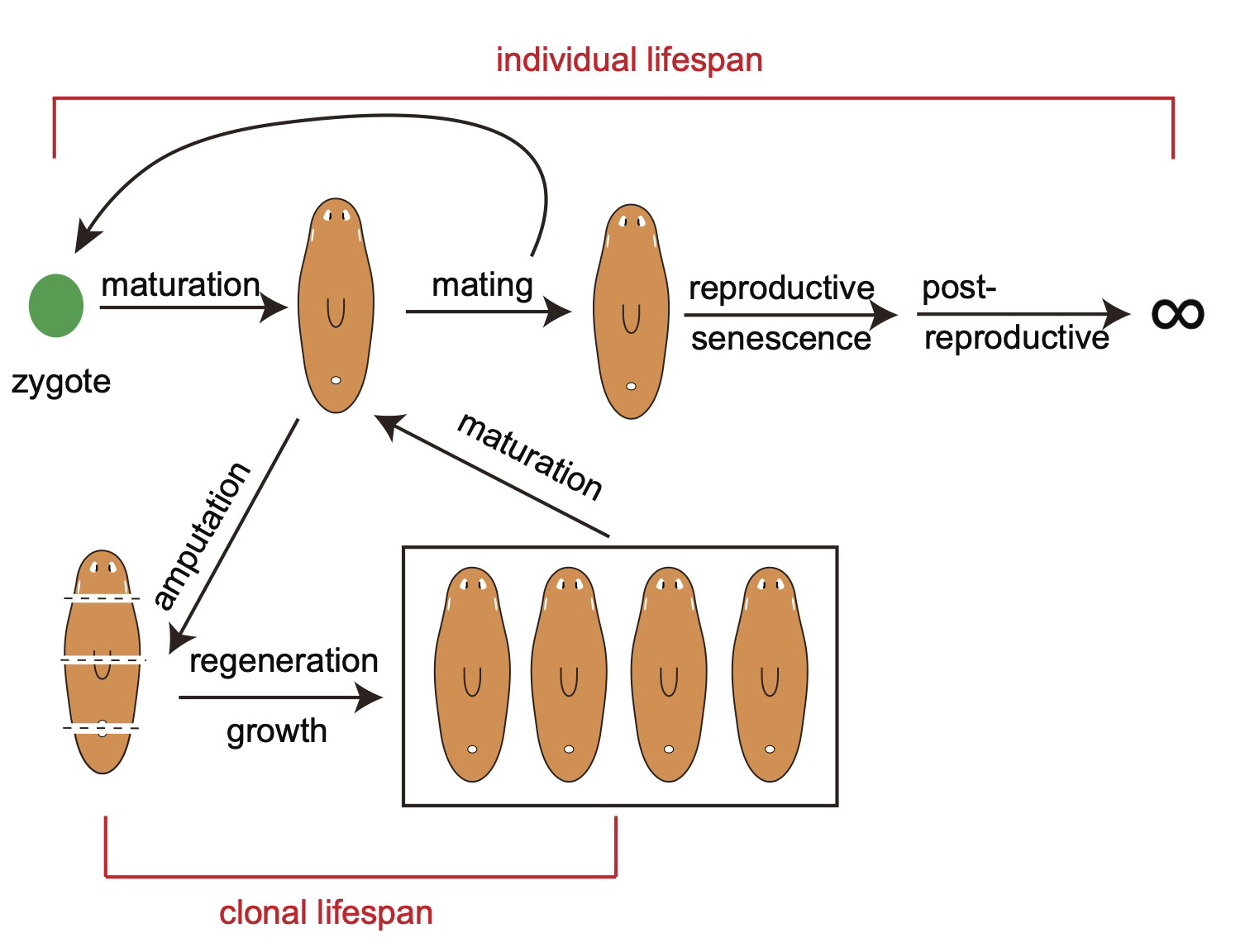
Species such as planarians expand our horizons of imagination and fuel innovation. The ability to regenerate any tissues lost to injury has fascinated many generations of biologists studying regenerative biology. Recent experimental data have shown that regeneration in older planarians can reverse age-associated physiological decline, effectively rejuvenating the animals and making them biologically younger. The remarkable biology manifested by planarians, encompassing whole-body regeneration and rejuvenation, intersects with some of the most critical topics of twenty-first-century research, including stem cell function, lifespan regulation, and healthspan improvement, despite being viewed by some as an evolutionary oddity. Here, we discuss how advances in next-generation sequencing technologies and the advent of genomic approaches over the past two decades have revolutionized planarian research. The results of these studies have transformed our understanding of regeneration, tissue patterning, germ cell development, chromosome evolution, aging, and age reversal (rejuvenation). We anticipate that genetic and genomic tools will drive groundbreaking discoveries in the fundamental mechanisms of regeneration, aging, and rejuvenation in the coming decades.
Planarian Aging and Rejuvenation (2025)
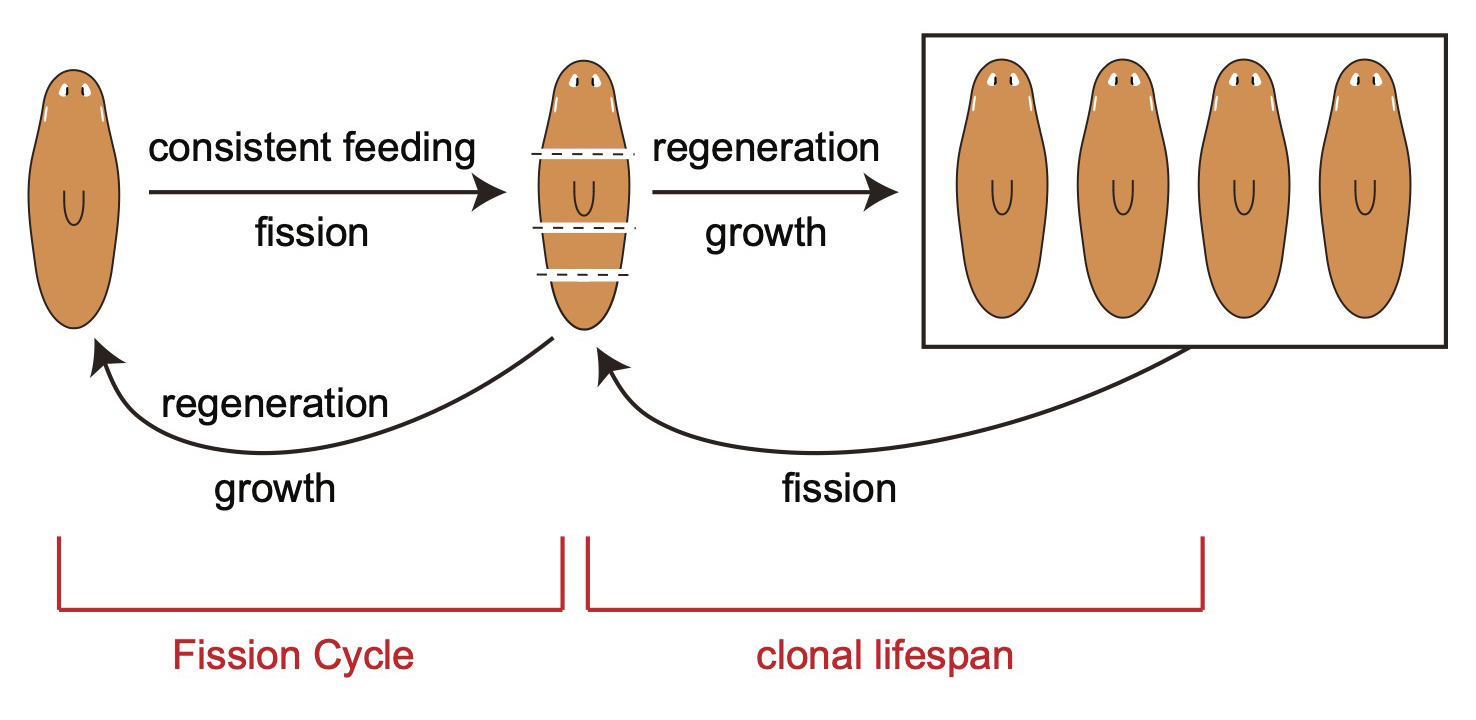
Aging, regeneration and whole-body rejuvenation
Dai X, Guo L
Research Briefing
Nature Aging (2025)
full text (no subscription needed)
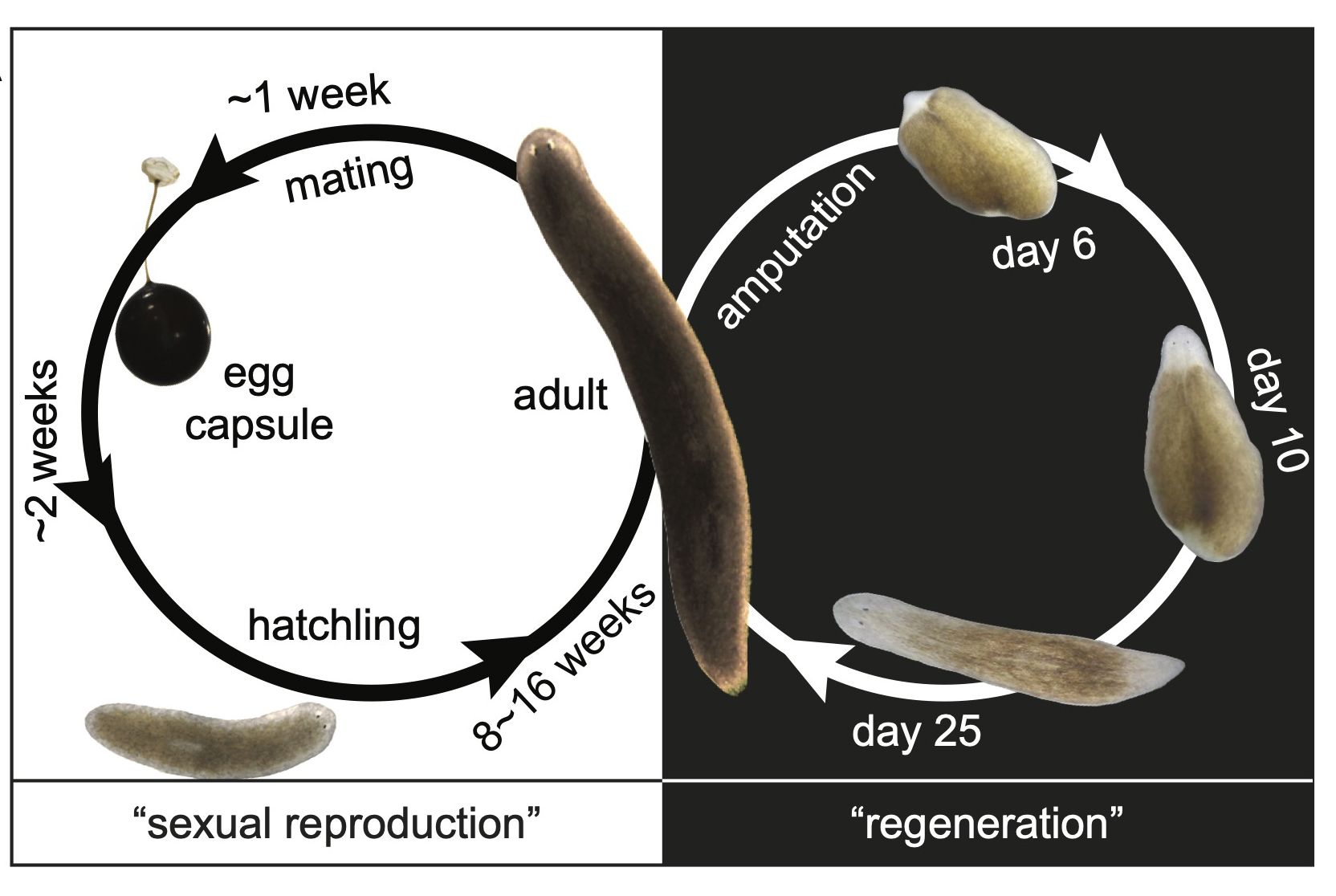
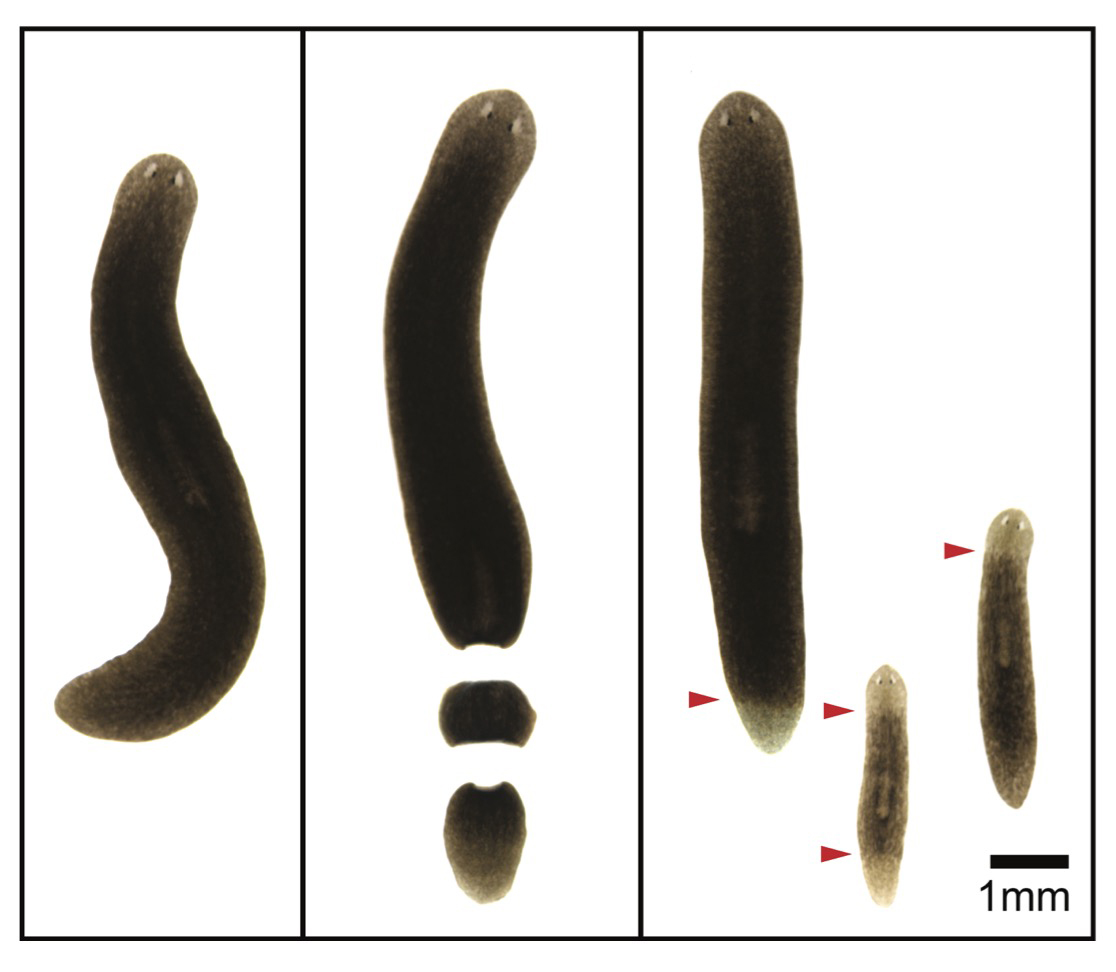
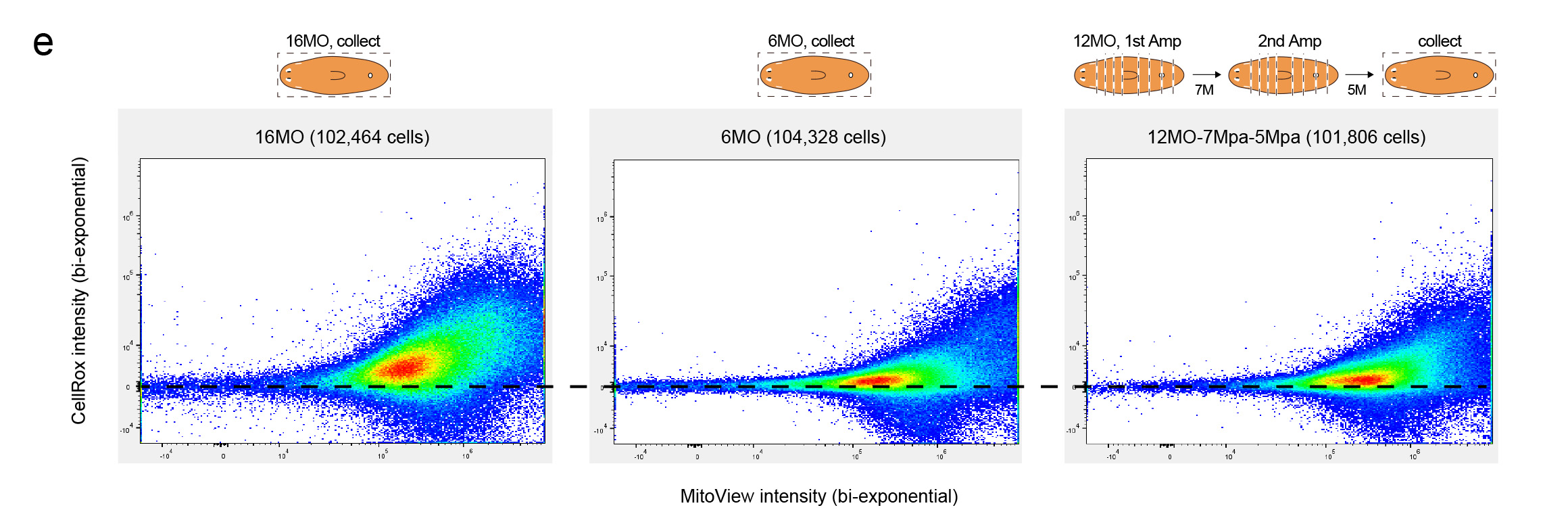
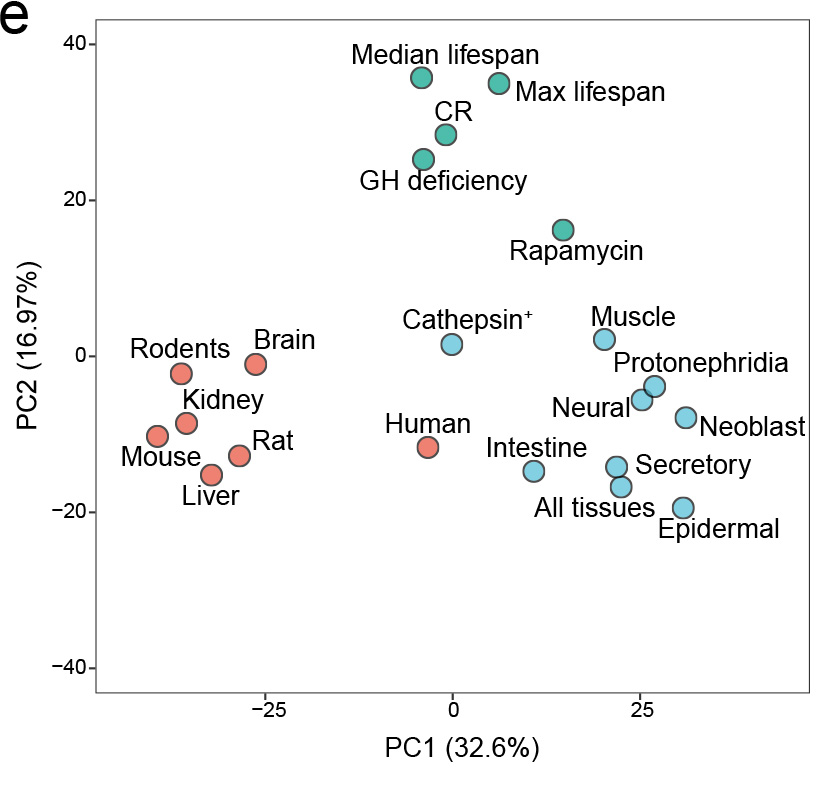
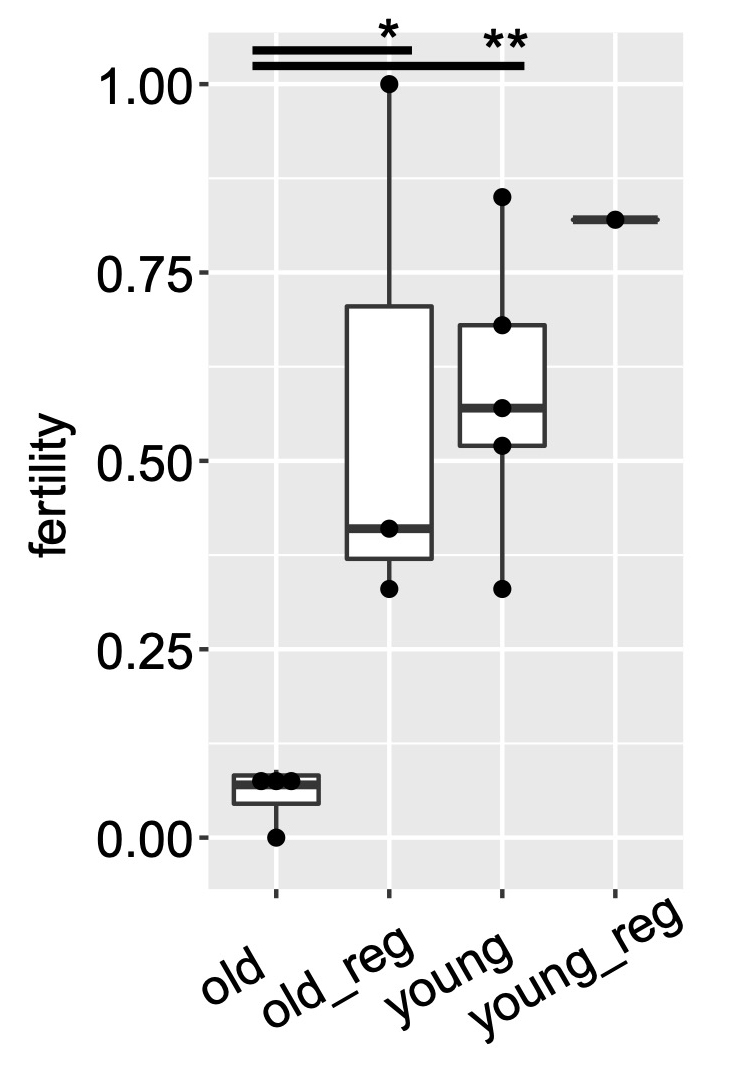
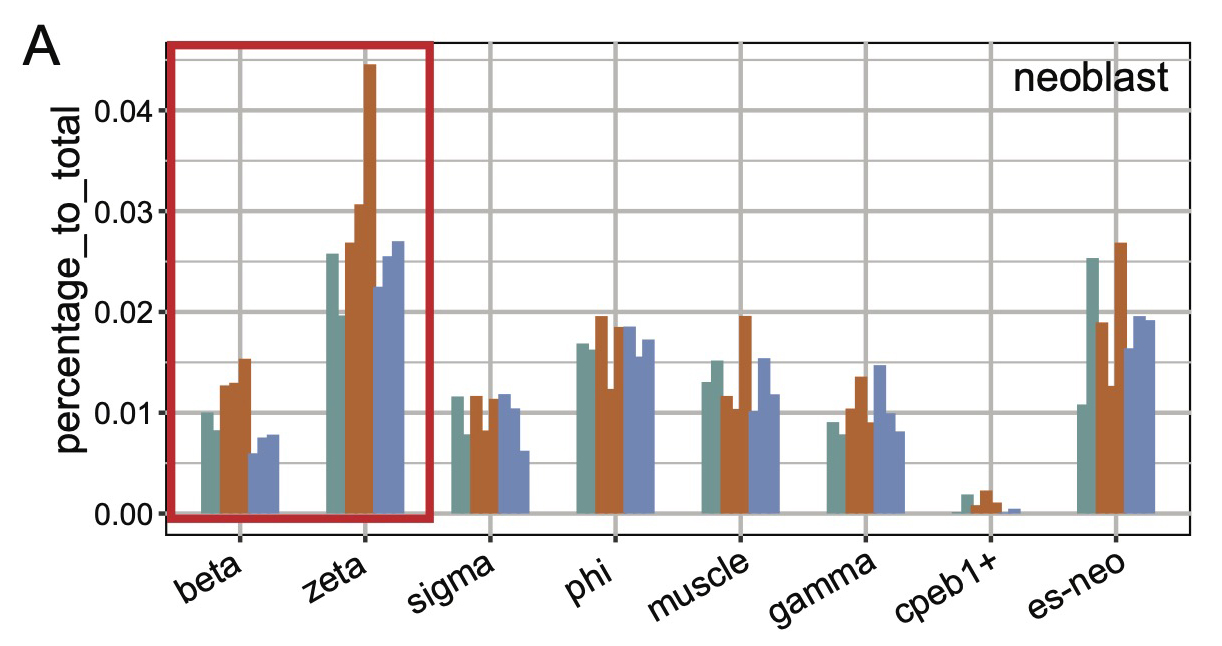
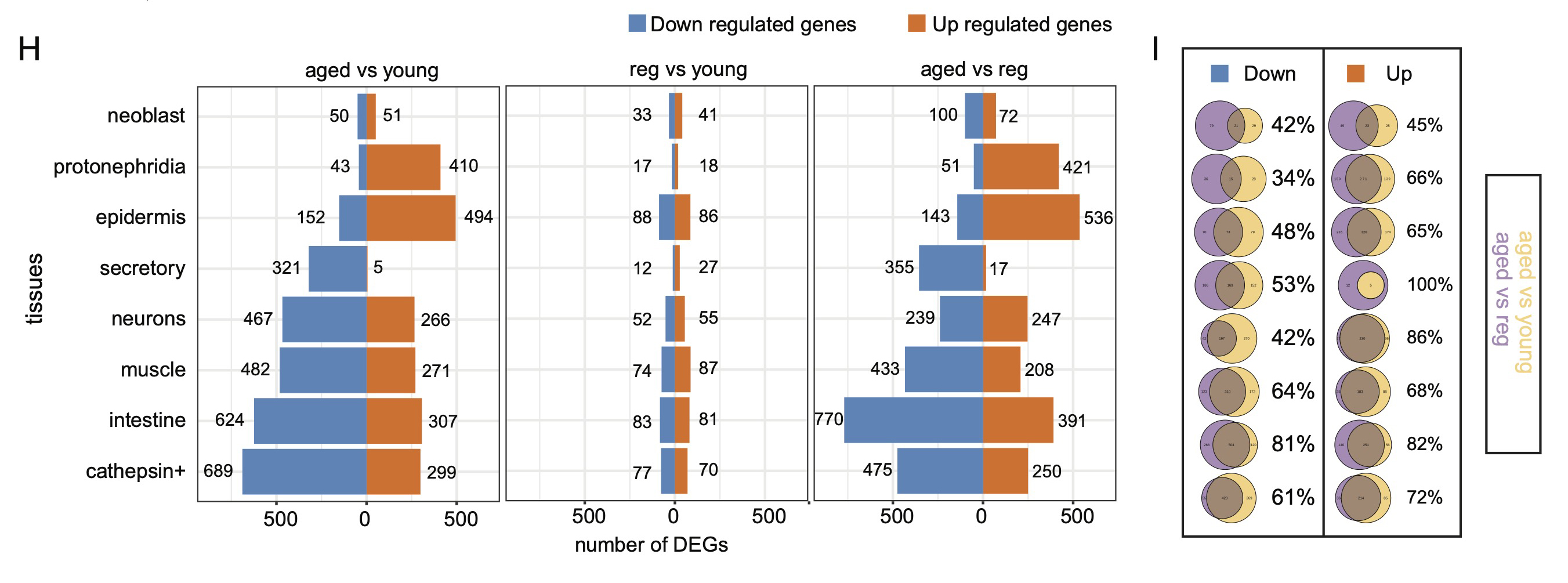
We identified aging-associated changes in sexual strains of the extremely long-lived planarian Schmidtea mediterranea. Following amputation and regeneration in older animals, aging-associated phenotypes were reversed to youthful states in both newly regenerated tissues and in tissues distant from the injury site that did not undergo regeneration, which resulted in global rejuvenation.
Planarian Aging and Rejuvenation (2025)
Regeneration leads to global tissue rejuvenation in aging sexual planarians
Xiaoting Dai, Xinghua Li, Alexander Tyshkovskiy, Cassandra Zuckerman, Nan Cheng, Peter Lin, David Paris, Saad Qureshi, Leonid Kruglyak, Xiaoming Mao, Jayakrishnan Nandakumar, Vadim N. Gladyshev, Scott Pletcher, Jacob Sobota &
Longhua Guo
Nature Aging (2025)
full text (open access)
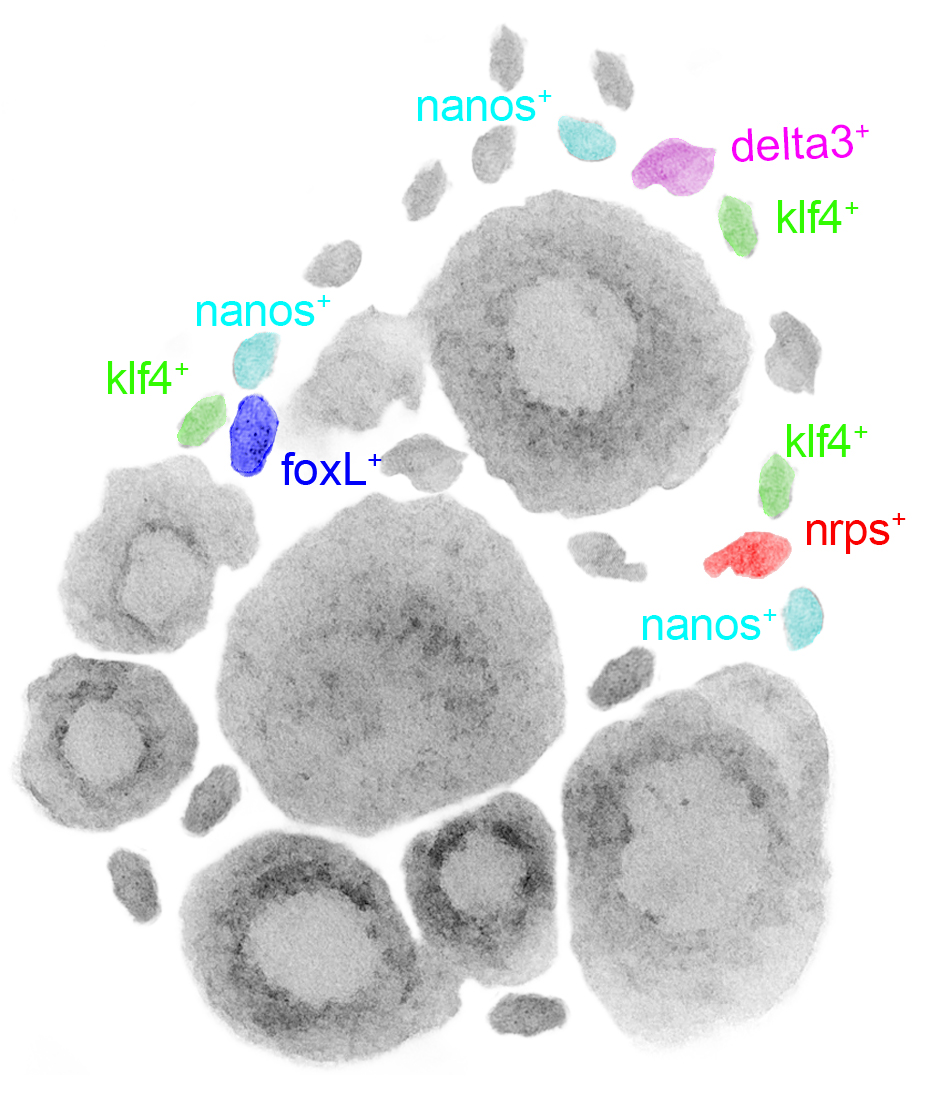
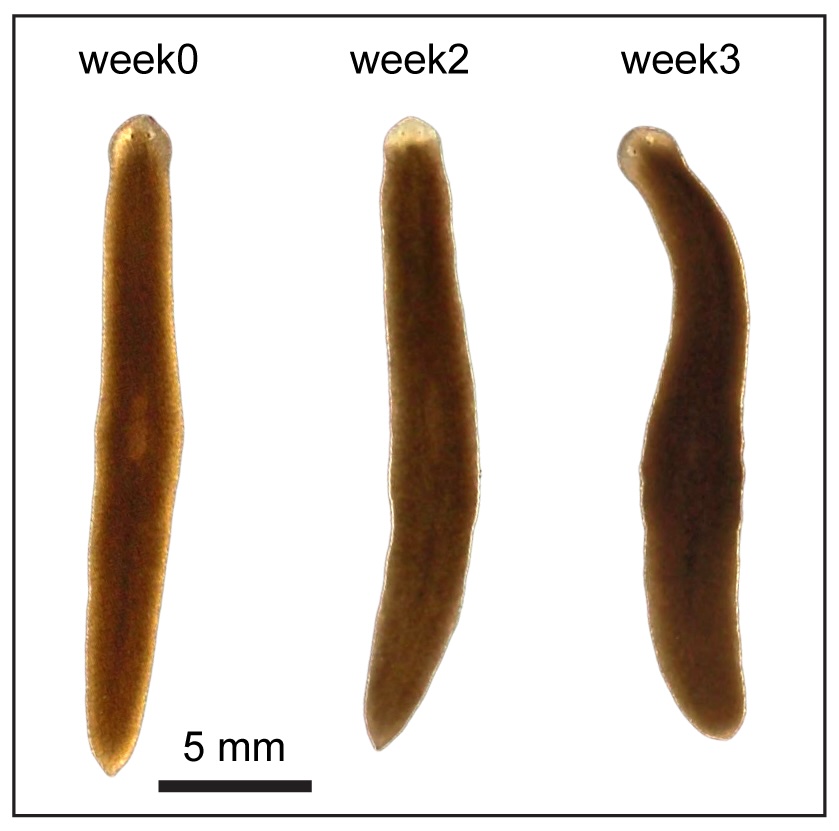
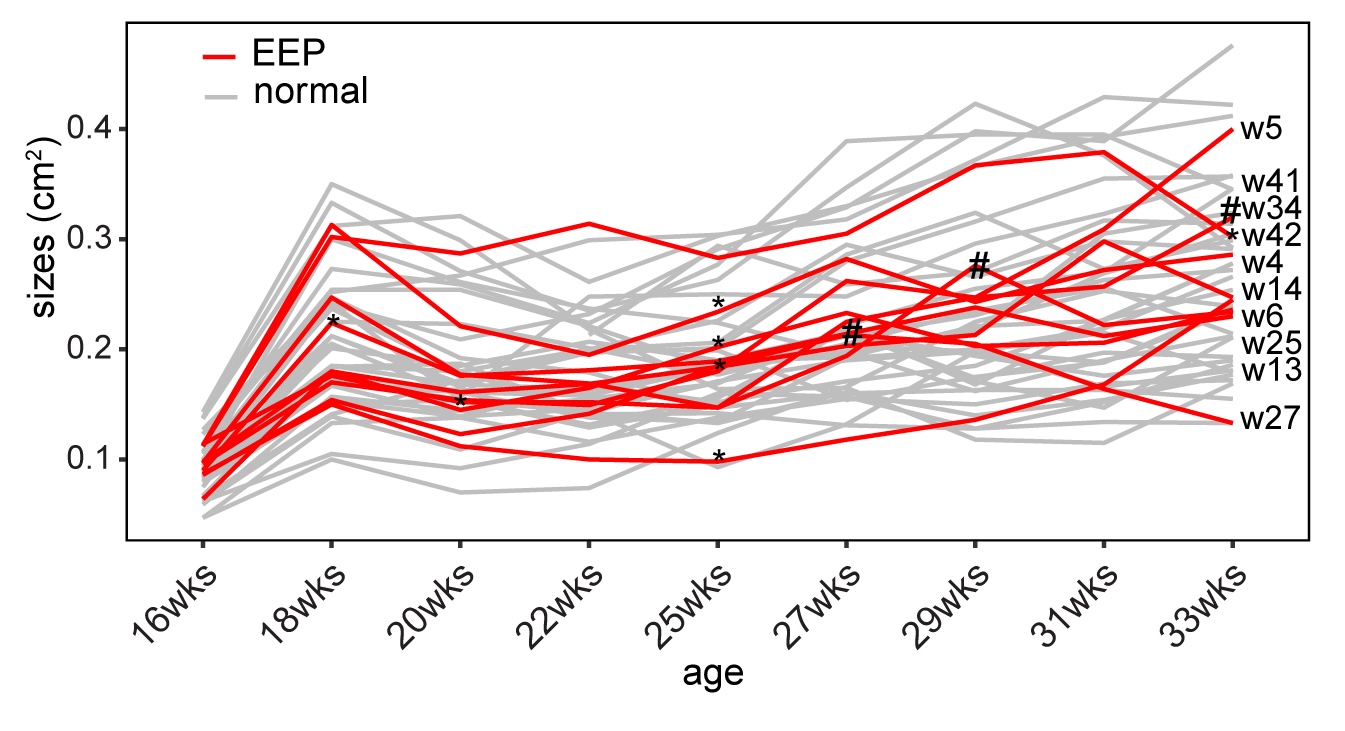
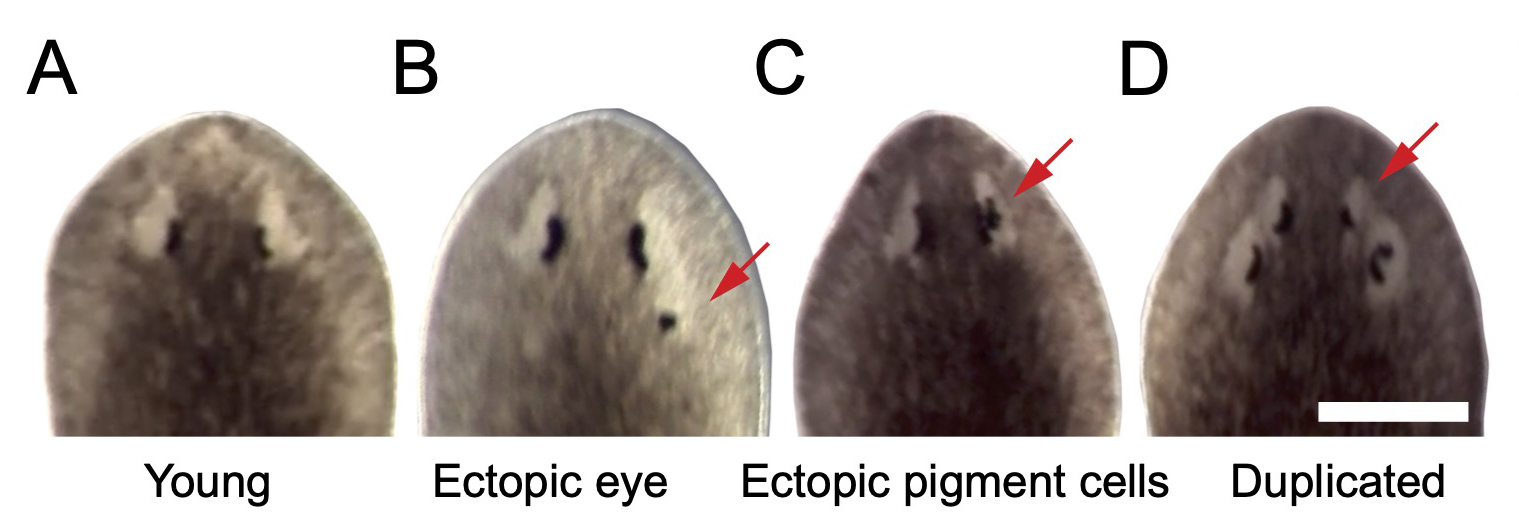
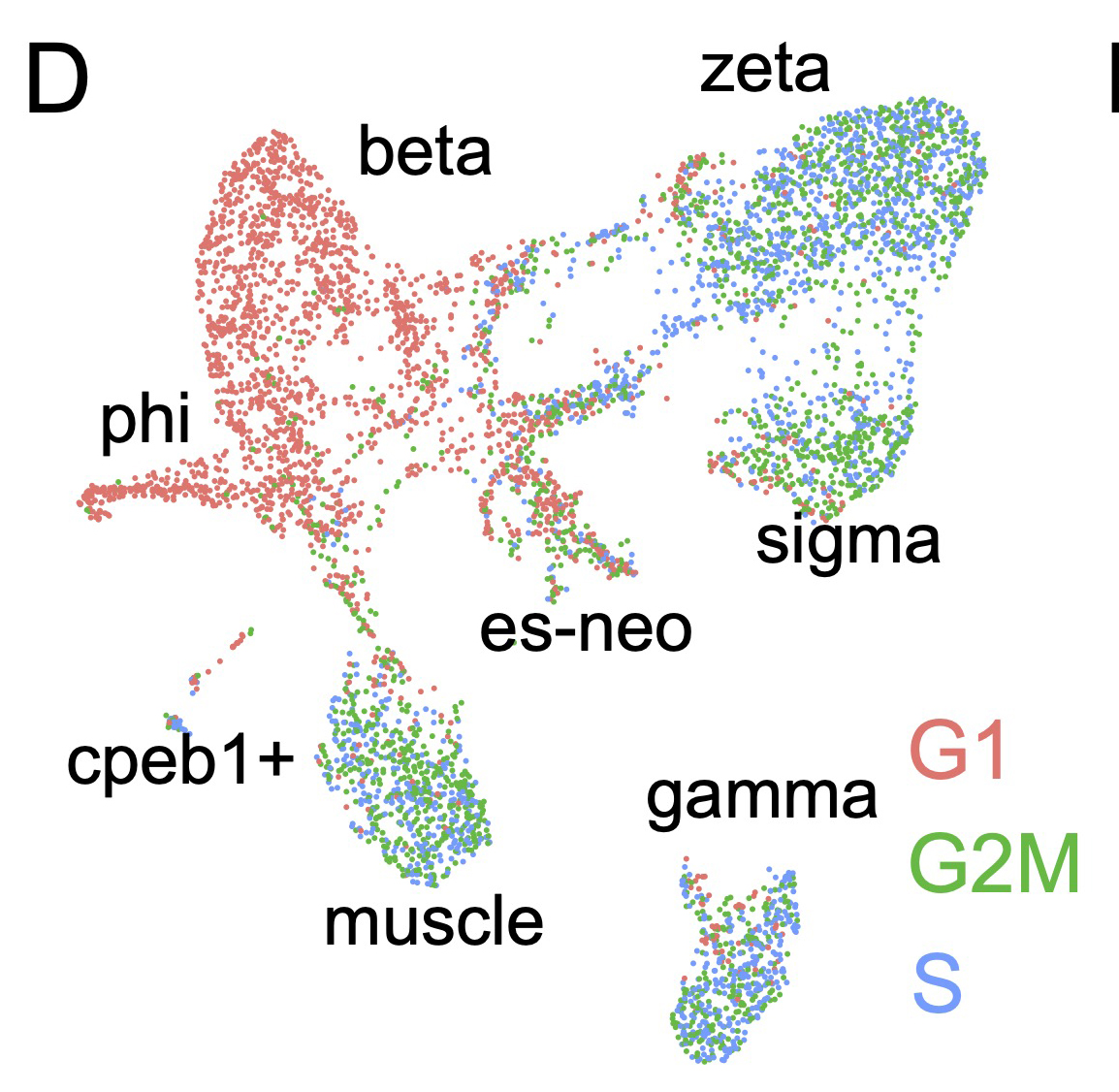
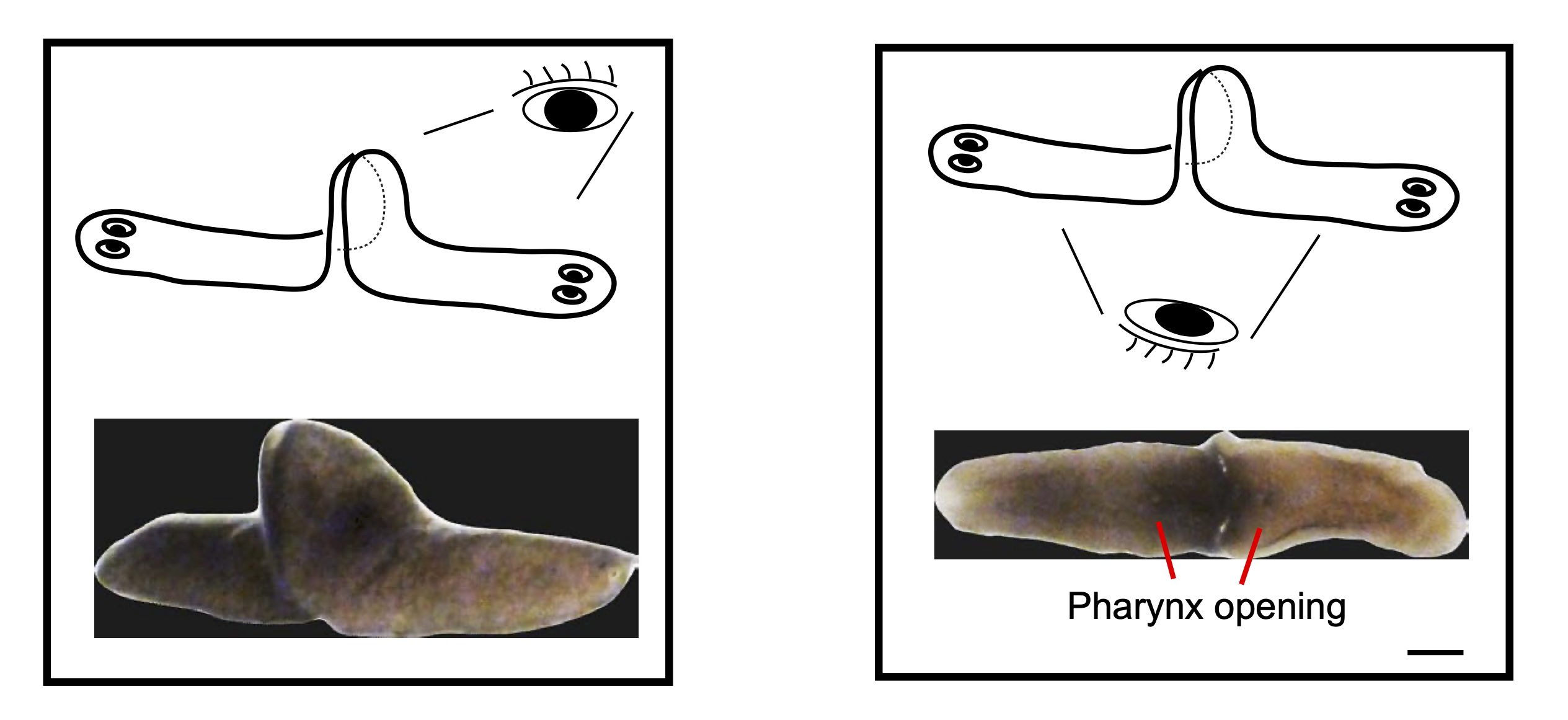
The possibility of reversing the adverse impacts of aging could significantly reduce age-related diseases and improve quality of life in older populations. Here we report that the sexual lineage of the planarian Schmidtea mediterranea exhibits physiological decline within 18 months of birth, including altered tissue architecture, impaired fertility and motility, and increased oxidative stress. Single-cell profiling of young and older planarian heads uncovered loss of neurons and muscle, increase of glia, and revealed minimal changes in somatic pluripotent stem cells, along with molecular signatures of aging across tissues. Remarkably, amputation followed by regeneration of lost tissues in older planarians led to reversal of these age-associated changes in tissues both proximal and distal to the injury at physiological, cellular and molecular levels. Our work suggests mechanisms of rejuvenation in both new and old tissues concurring with planarian regeneration, which may provide valuable insights for antiaging interventions.
Planarian Aging and Rejuvenation (2023)
Age Reversal
Dai X, Li X, Pletcher S, Paris D, Kruglyak L, Sobota J, Guo L
Regeneration leads to global tissue rejuvenation in aging sexual planarians.
bioRxiv (2023)
full text
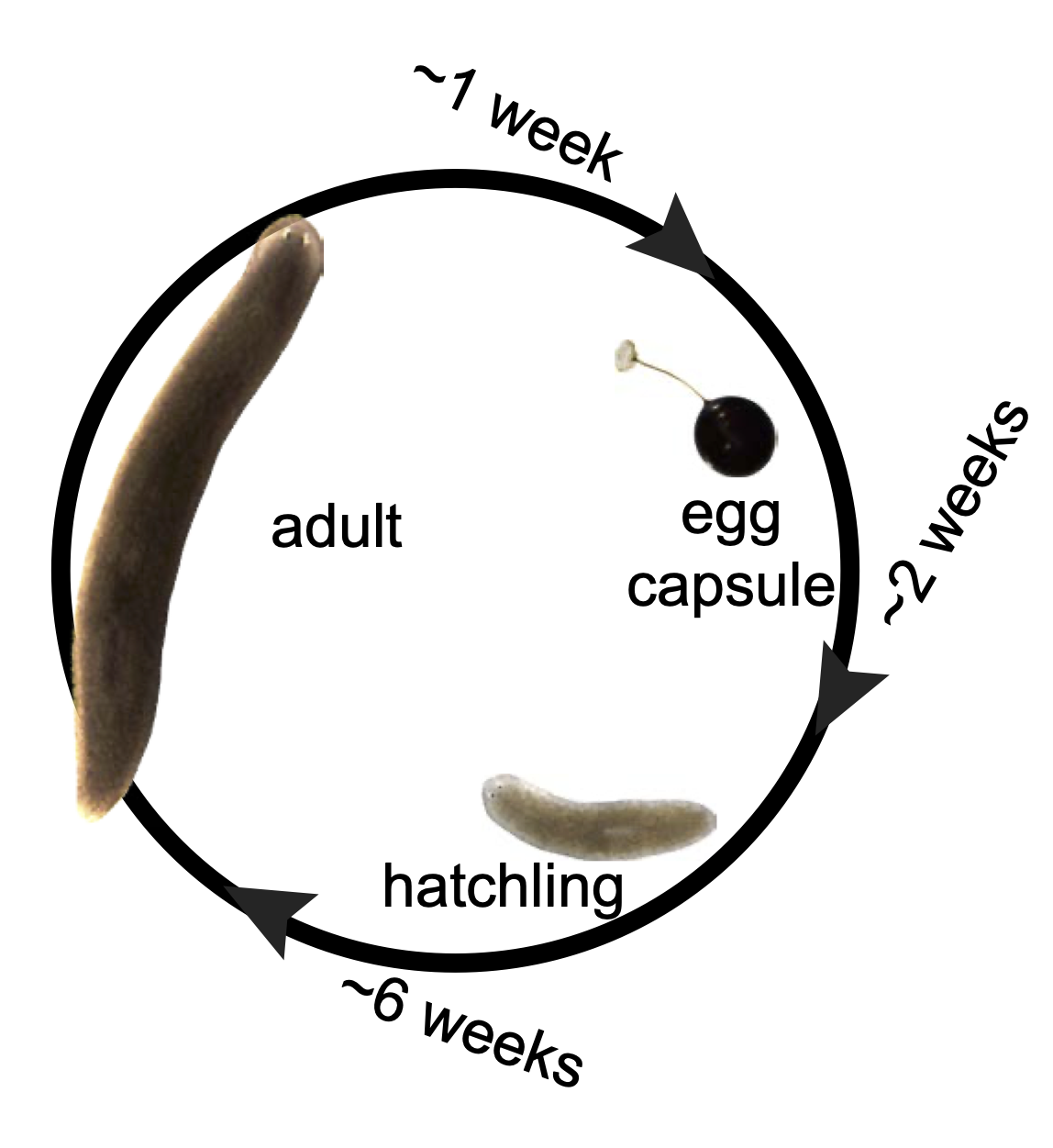
We defined chronological ages in the “immortal” sexual planarians based on their birth date, as researchers studying short-living models (flies, nematodes, fish, mice etc.). We found multiple aging traits within a year’s time. Most intriguingly, we found planarians are capable of whole-body age reversal.
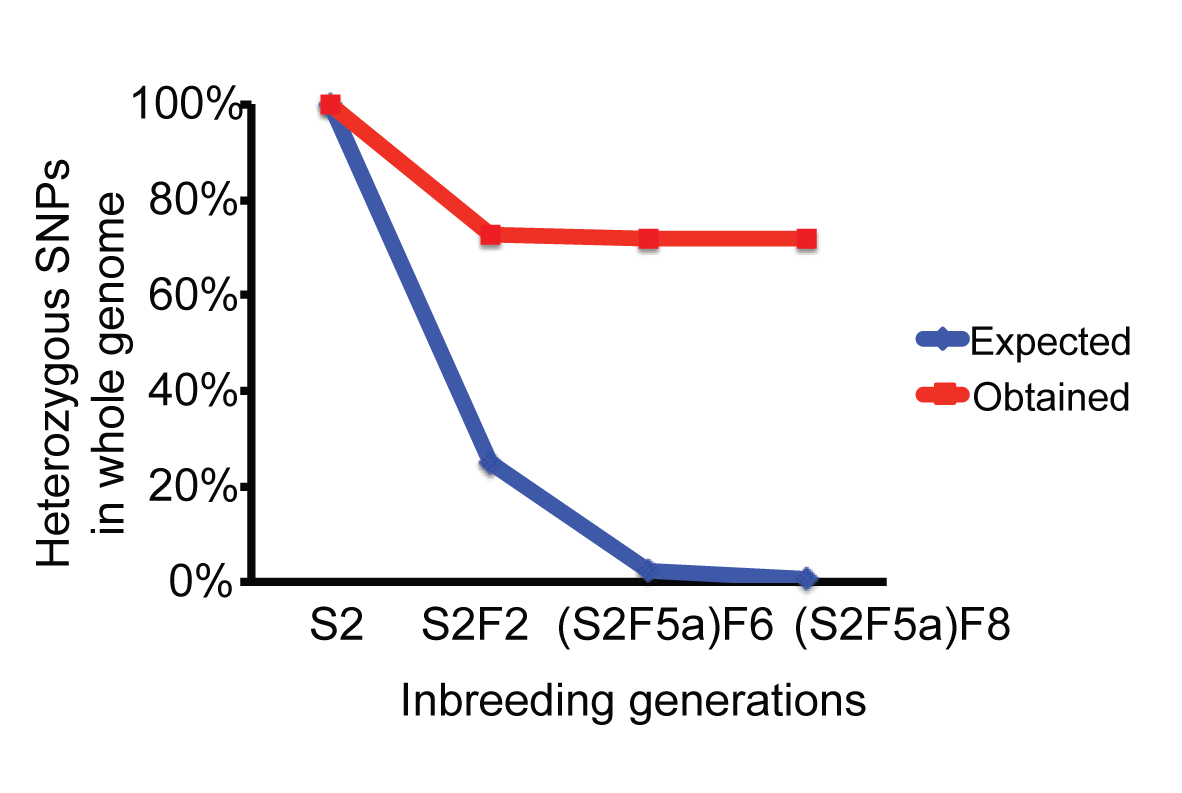
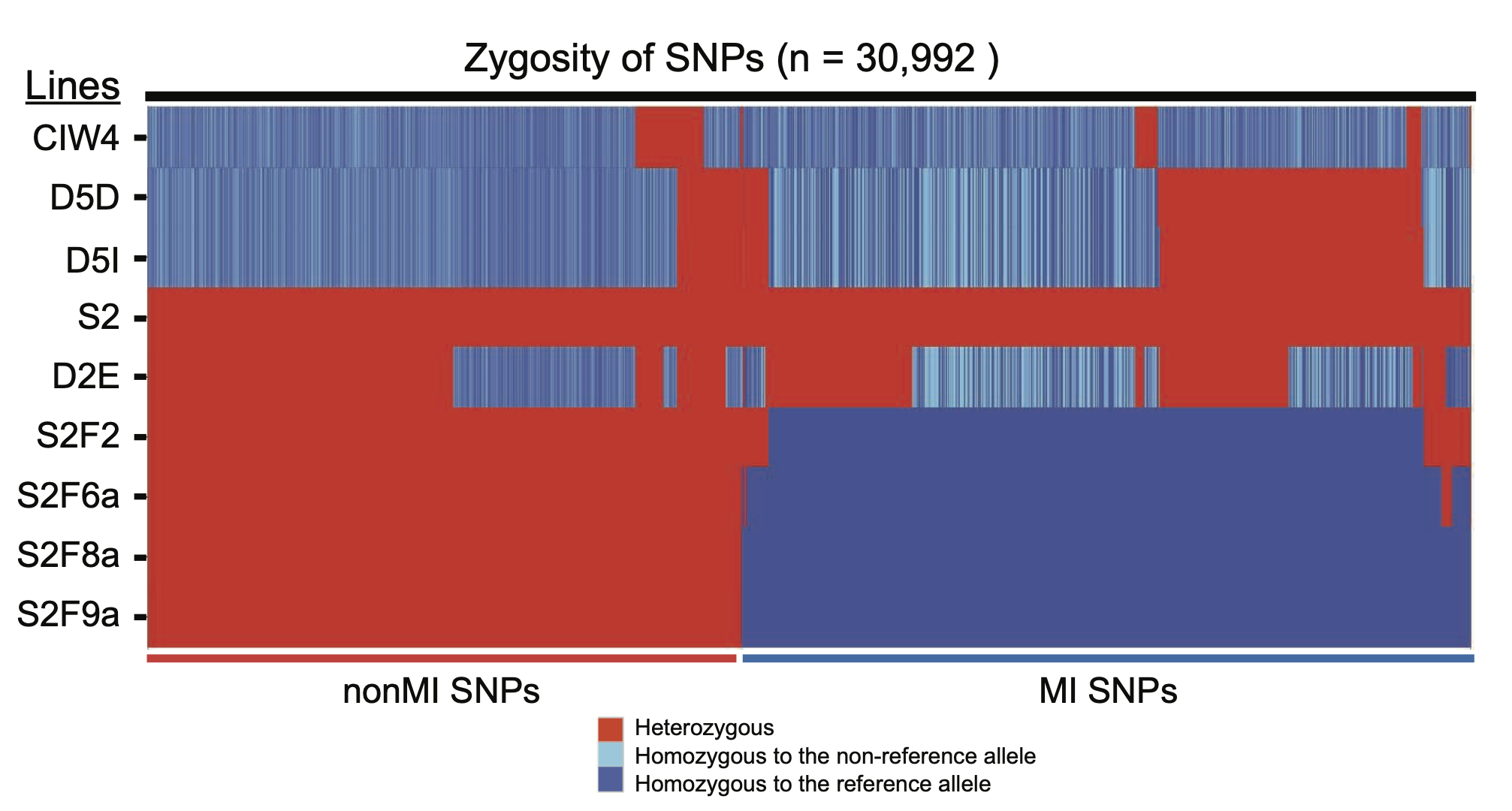
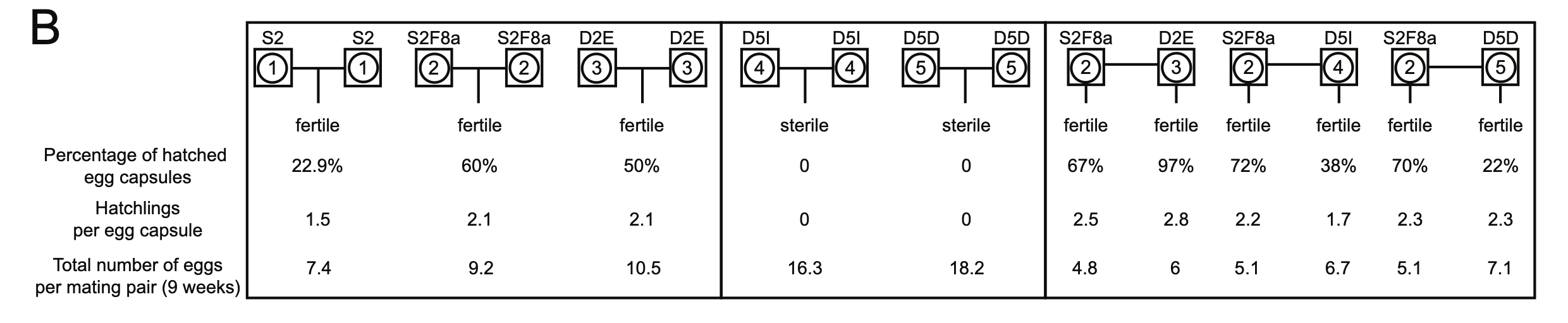
Planarian Genome and Genetics (2016)
Inbreeding
Guo L, Zhang S, Rubinstein B, Ross EJ, Sánchez Alvarado A, Widespread maintenance of genome heterozygosity in Schmidtea mediterranea. Nature Ecology & Evolution 1, 0019 (2016)
https://doi.org/10.1038/s41559-016-0019
We established robust procedures to have the sexual planarians produce hatchlings whenever we need them and established a 10-generation inbreeding pedigree, from which we uncovered a non-Mendelian phenomenon.
Planarian Genome and Genetics (2022)
Chromosome
Guo L#, Bloom J, Dols-Serrate D, Boocock J, David E, Schubert O, Kozuma K, Ho K, Warda E, Chui C, Wei Y, Leighton D, Vergara TL, Riutort M, Sánchez Alvarado A, Kruglyak L#. Island specific evolution of a sex-primed autosome in Schmidtea mediterranea. Nature June, 2022
# co-corresponding,
https://www.nature.com/articles/s41586-022-04757-3
biorxiv:10.1101/2021.05.24.445457
With single chromosome sequencing, we established a chromosome-scale genome assembly, from which we found the non-Mendelian inherited planarian genome is chromosome 1. Chromosome 1 has multiple features of an emerging sex chromosome. As sexual planarians are simultaneous hermaphrodites, we termed this chromosome a sex-primed autosome.
Gecko Genetic and Phenotypic diversity (2021)
Cancer metastasis
Guo L#, Bloom J, Sykes S, Huang E, Kashif Z, Pham E, Ho K, Alcaraz A, Xiao X, Duarte-Vogel S, Kruglyak L#. Genetics of white color and irridophoroma in “Lemon Frost” leopard geckos. PLoS Genet, 17(6): e1009580, (2021)
# co-corresponding https://doi.org/10.1371/journal.pgen.1009580
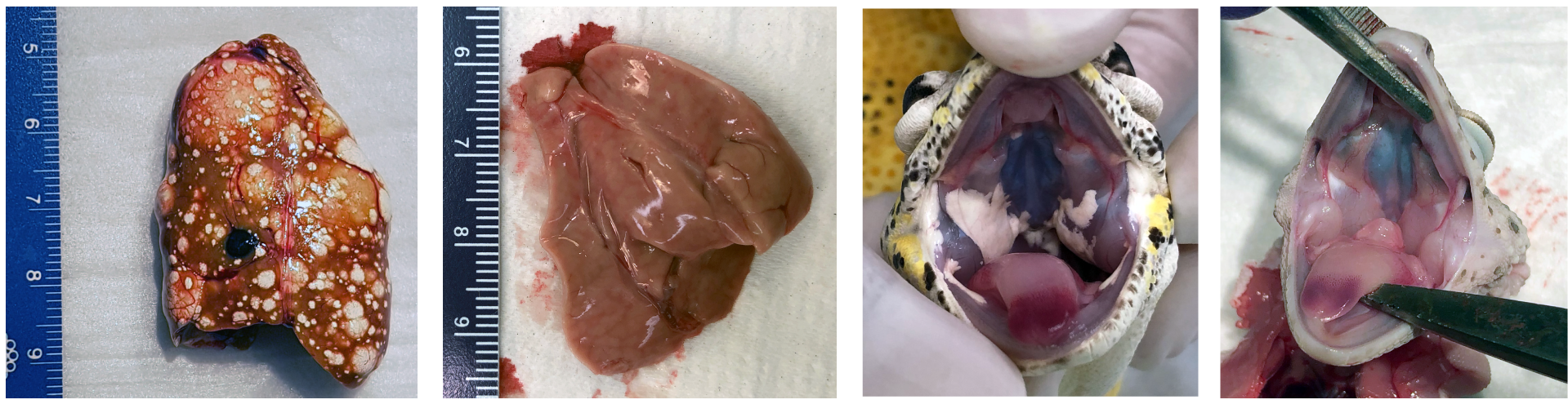
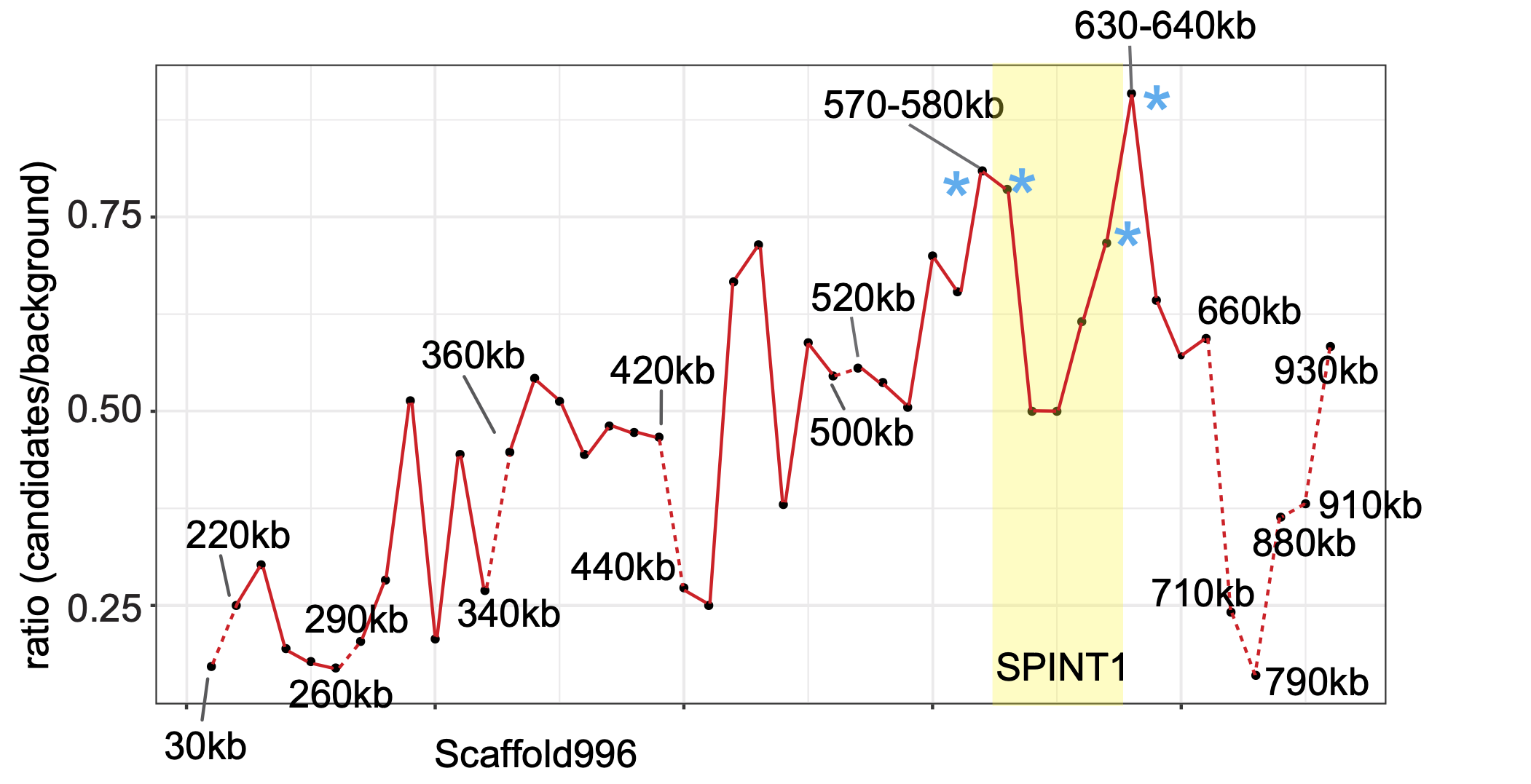
The skin cancer in Lemon Frost geckos spontaneously metastasize to the liver. With whole genome sequencing and RAD sequencing, we mapped the genetic loci that caused the Lemon Frost phenotype to a tumor suppressor, SPINT1.
Featured by HHMI News, the Scientist, Scientific America, Popular Science etc.
Publication listed by years
2025
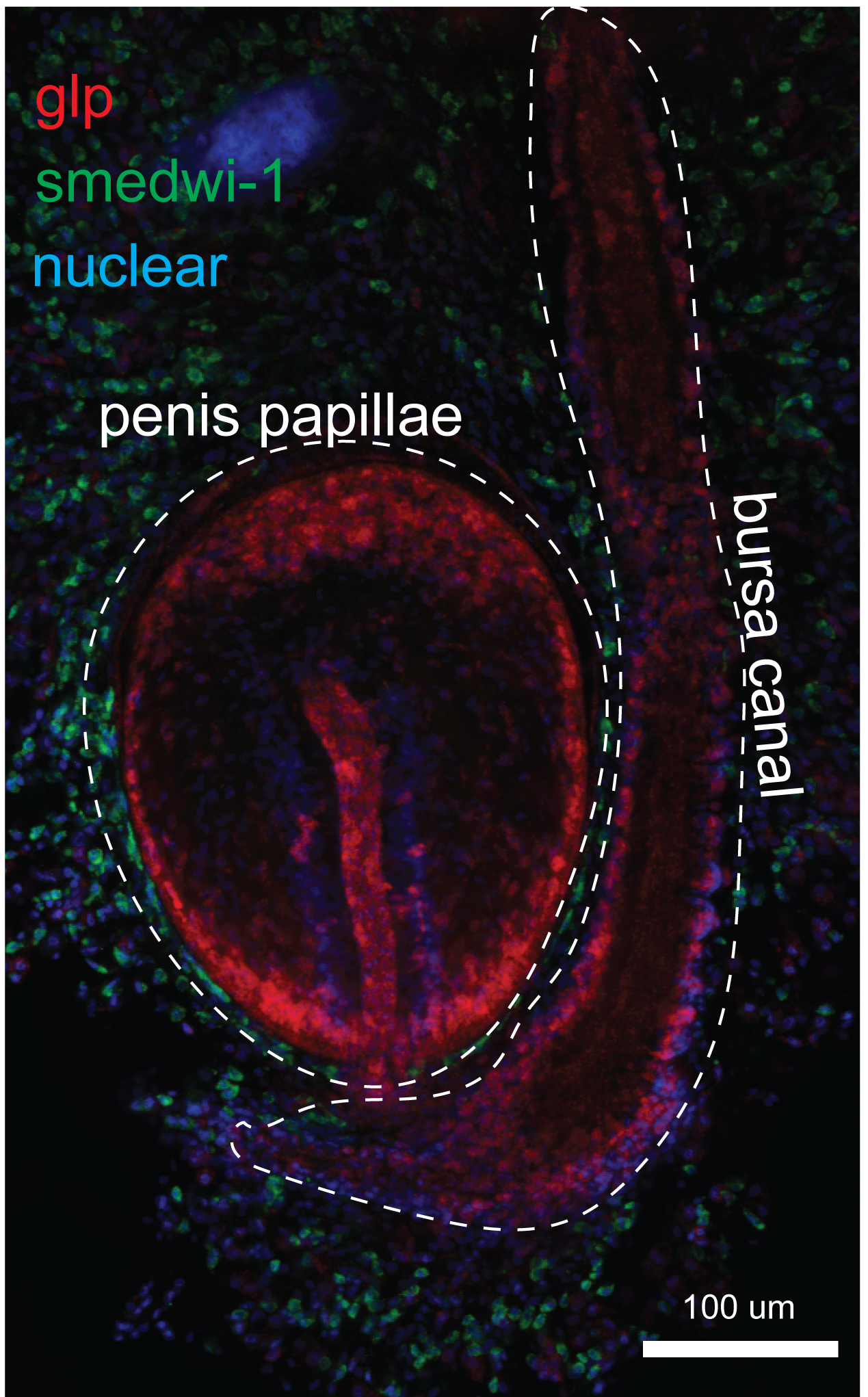
Oogenesis involves a novel nuclear envelop remodeling mechanism in Schmidtea mediterranea
Developmental Biology (2025) 520:13-20, doi: 10.1016/j.ydbio.2024.12.018
Longhua Guo, Fengli Guo, Shasha Zhang, An Zeng, Kexi Yi, Melainia McClain, Claus-D. Kuhn, Tari Parmely, Alejandro Sánchez Alvarado
2024
Developmental defects in planarian hatchlings (Platyhelminthes: Rhabditophora)
Zoologia (2024) doi: 10.1590/S1984-4689.v41.e23087
Xiaoting Dai, Jacob Sobota, Xinghua Li, Longhua Guo
Extraordinary model systems for regeneration
Development (2024) 151 (20): dev203083. doi: 10.1242/dev.203083
Alice Accorsi, Longhua Guo, Wallace F Marshall, Mathilda Mommersteeg, Yuichiro Nakajima
2023
Genetics and biology of coloration in reptiles: the curious case of the Lemon Frost geckos
Physiological Genomics (2023) 55(11): 479-486. doi:10.1152/physiolgenomics.00015.2023
Longhua Guo, Leonid Kruglyak